SEARCH
PRODUCT
PRODUCT PARAMETERS
Ammonium sulfate, commonly known as ammonium sulfate, is an inorganic salt compound with the chemical formula (NH4)2SO4 and a molecular weight of 132.14. It appears as colorless rhombic crystals at room temperature. Industrial products generally appear as small white or slightly yellowish crystals, but a few byproducts have colors such as slightly green or dark brown. Its relative density is 1.77 and its melting point is 280°C (decomposition). It is freely soluble in water but insoluble in ethanol and acetone. Its aqueous solution is acidic (pH = 5.5, 0.1 mol/L), and its solubility in water decreases with increasing ammonia content. Ammonium sulfate is hygroscopic and solidifies into lumps after absorbing moisture. Its chemical properties reflect those of ammonium salts and sulfates. It reacts with alkali to release ammonia gas and decomposes upon heating into ammonia, nitrogen, and sulfur dioxide. It reacts with barium chloride to form a barium sulfate precipitate and reacts with alkaline potassium mercuric iodide solution to form a brown precipitate.
In industry, ammonium sulfate is primarily produced by the direct reaction of ammonia with sulfuric acid or by passing ammonia and carbon dioxide into a suspension of gypsum powder. Ammonium sulfate contains approximately 21% nitrogen and is a fast-acting fertilizer suitable for general crops. It can be used in the salting-out process in the pharmaceutical industry, in yeast culture in fresh yeast production, as a solder, a fabric fire retardant, a catalyst for food coloring, a dyeing auxiliaries for acid dyes, and a leather deliming agent.
PRODUCT USES
This excellent nitrogen fertilizer (commonly known as fertilizer powder) is suitable for general soils and crops. It promotes vigorous foliage growth, improves fruit quality and yield, and enhances crop resistance to disasters. It can be used as a base fertilizer, topdressing, and seed fertilizer. It reacts with salt through a double decomposition reaction to produce ammonium chloride, reacts with aluminum sulfate to produce ammonium alum, and is combined with boric acid and other materials to manufacture refractory materials. It can be added to electroplating baths to increase conductivity. It is also a catalyst for food coloring, a nitrogen source for yeast culture in fresh yeast production, a dyeing auxiliaries for acid dyes, and a leather deliming agent. It is also used in beer brewing, chemical reagents, and battery production. Another important function is the mining of rare earths. Ammonium sulfate is used as the raw material, and ion exchange is used to exchange the rare earth elements in the ore. The leaching liquid is then collected, impurities are removed, precipitated, pressed, and burned to form rare earth ore.

FACTORY DISPLAY
FAQ
Q: Are you a factory or a trading company?
A: We are a company integrating manufacturing and trading.
Q: Can you provide samples for testing before mass production?
A: Of course. We can send you samples free of charge, and we also include a COA. However, you will be responsible for the shipping fee.
Q: How can I get an accurate quote?
A: Please provide the exact product specifications, particle size, color, and intended use, and we will provide you with an accurate quote.
Q: Can you provide a COA?
A: Of course, but only for standard products. If you require an accurate COA, we will produce and test samples before providing you with an accurate COA.
Q: Do you accept ODM or OEM services?
A: Yes, we offer customized services for ingredients, packaging, and labeling. We have strong design capabilities. Please contact us to design your own brand.
Q: Can I visit your factory before placing a large order?
A: Of course. We welcome you at any time.
Q: What is the loading port?
A: Usually Qingdao or Tianjin.
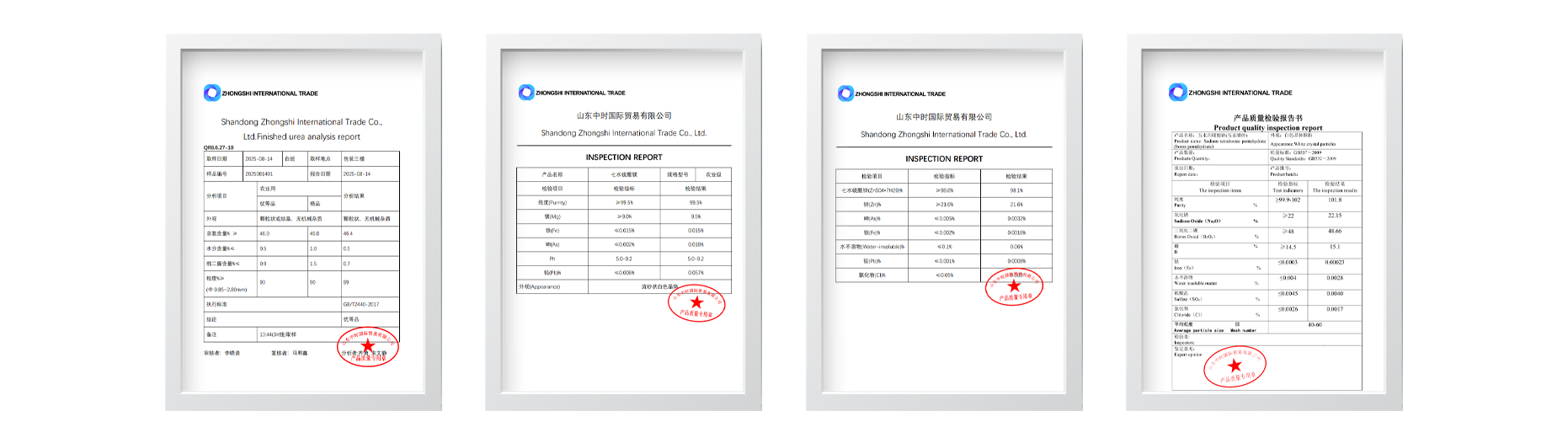
Test certificate
CONTACT US
PRODUCT RECOMMENDATION
Professional Service
Interested? Leave your contact details.
Search Starts Here





















